With so many countries under lockdown, researchers from around the world are racing against time to develop a vaccine for COVID-19. Keeping with our theme of DNA, we explore the topic of DNA vaccines and their potential for battling the COVID-19 pandemic.
The purpose of a vaccine
Immunity is the key to protecting the body against foreign pathogens such as bacteria and viruses. Upon infection, the immune system will recognize a pathogen as foreign and mount an immune response to destroy the pathogen. Antibodies perform the essential ‘recognition’ component of the immune response. However, before antibodies can be utilized for recognition, they must be produced by specialized immune cells within our bodies.
Infections are the natural way to produce antibodies. For instance, if a person contracts chickenpox, a disease caused by the varicella-zoster virus, their immune cells will learn to generate antibodies against this virus. If there is a subsequent exposure to the same virus, the body will recognize the varicella-zoster virus and destroy it before it can lead to chickenpox. This person thus has acquired immunity against chickenpox.
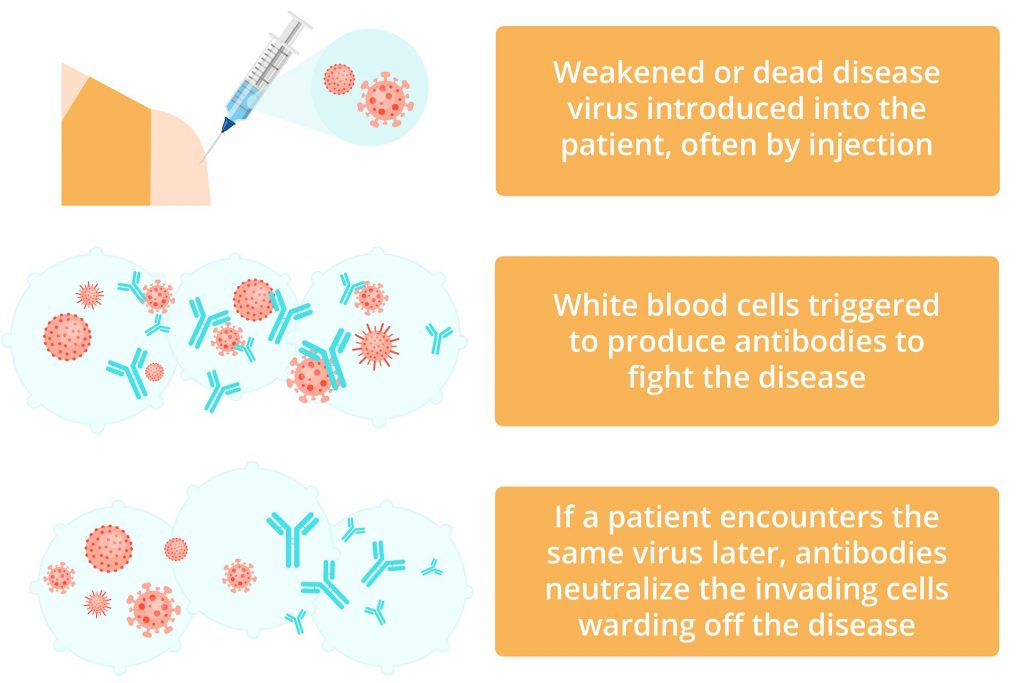
Vaccines offer an alternative avenue for developing antibodies. When a person is vaccinated, an altered form of the pathogen (e.g. a dead or weakened virus, a surface protein of the virus, or a toxin produced by a virus) is injected into the body to mount an immune response.
The immune system will respond by generating antibodies against the specific pathogen present in the vaccine without actually causing the disease.
Vaccines have greatly improved our ability to effectively manage infectious diseases. Effective vaccination has dramatically reduced the incidents of certain debilitating diseases (e.g. polio) and has played a critical role in eradicating smallpox.
Why use a DNA vaccine?
Therapeutic vaccines can be developed using several different methods. DNA-based vaccines make use of DNA to deliver a specific component of a pathogen into cells. Many studies have explored the utility of DNA vaccines in the early 1990s and the 2000s due to the following advantages:
Firstly, DNA vaccines are safer than using live-attenuated (weakened) viruses because there is no risk of infection. Attenuated vaccines carry the active pathogen, which has been modified to reduce its virulence or the ability to cause disease. This means there is still a chance of infection.
Along the same line, because DNA vaccines only carry the minimal components necessary to produce an immune reaction (aka a subunit vaccine), they are associated with minimal side effects. Lastly, DNA vaccines are also easier to produce, cheaper, and can be stored at room temperature for a longer period of time.
These advantages and the need for a rapid response to battle COVID-19 has positioned DNA vaccines as a front-line candidate for tackling this pandemic.
A brief introduction to coronaviruses
In recent years coronaviruses have emerged as one of the major pathogens that cause respiratory disease outbreaks. SARS-CoV and MERS-CoV are just two examples of viral outbreaks that lead to many deaths in the last 20 years.
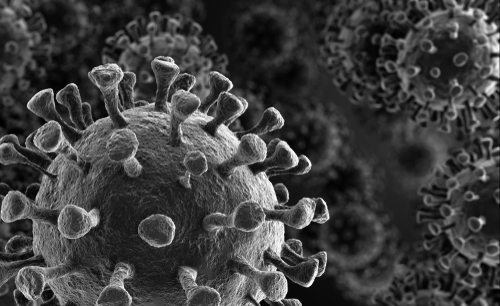
Coronaviruses are a large family of viruses with characteristic club-like spikes that project out from the surfaces of their coats. They can infect many different animals and are particularly dangerous because they can cross species barriers, meaning they can transfer from animals to humans. For instance, SARS-CoV is thought to be an animal virus that crossed the species barrier (possibly from bats) to infect humans in 2002.
In most cases, the symptoms of a coronavirus infection can be similar to those of the common cold. But in some instances, as we are experiencing currently with COVID-19, it can lead to severe respiratory symptoms.
Fortunately, the strict social distancing measures adopted by many countries around the world have effectively reduced the spread of COVID-19. However, there is still a need for preventing future outbreaks, for which immunization may hold the key.
Where are we with DNA vaccines against COVID-19?
According to the latest reports from the World Health Organization (WHO), seven clinical trials are currently underway to test the safety and feasibility of COVID-19 vaccine candidates.
One of these trials will test the INO-4800 DNA vaccine designed by Inovio Pharmaceuticals Inc., an American-based biotechnology company, in a phase I clinical trial (NCT04336410). Two doses of the DNA vaccine will be injected into 40 healthy adult volunteers four weeks apart to determine the vaccine’s safety and ability to generate an immune response.
WHO lists two additional DNA vaccines in preclinical stages, one by Cobra Biologics and another by Takara Bio.
The need for a rapid response
COVID-19 has had a tremendous impact on the global economy, societies, and politics. As such, there is an extraordinary need to develop a vaccine to combat this pandemic. Given the immensity of this challenge and the necessity for a rapid response, it is likely that we will not find “the solution” to tackle COVID-19.
Instead, perhaps we need to take an approach similar to that of Hippocrates where “for extreme diseases, extreme methods of cure, as to restriction, are more suitable”. With multiple vaccines, we may find it easier to bring at least a small semblance of ‘normalcy’ back to our daily lives.
References:
- Coronaviruses: An Overview of Their Replication and Pathogenesis. (doi: 10.1007/978-1-4939-2438-7_1)
- Engineering DNA vaccines against infectious diseases. (doi: 10.1016/j.actbio.2018.08.033)
WHO COVID Vaccine trials list – https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf